Happening Now
Please continue to watch Happening Now for commercial and FEP updates.
For Medicare Advantage updates please reference the Medicare Advantage page.
Feb. 11, 2026 - Universal PA Request Form: Update to Submitting Medical Records for Preauthorizations
We are excited to announce, effective immediately, all medical records and any required documentation for commercial preauthorization (PA) requests submitted via the Universal PA Request (BCBSNE) form must now be uploaded online. This includes medical records, clinical notes and any additional information needed to support the request.
Faxed documentation will no longer be accepted. Submitting all materials together ensures faster review, reduces administrative delays and helps prevent incomplete submissions.
If the PA was originally submitted through NaviNet, any additional documentation must be uploaded directly in NaviNet, not through the Universal PA Request form.
Submitting Additional Documents via Universal PA Request form
- Go to the Universal PA Request BCBSNE Form on the Preauthorization page.
- Select Upload Documents.
- Enter the Auth ID or LB77 ID, to retrieve the PA.
- Provide your name and phone number.
- Verify authorization details: NPI, Member ID, Member Name and DOB.
- Upload supporting documents (up to 10MB per file) and add notes if needed.
- Submit and watch for the confirmation message.
When to Use the Universal PA Request Form
Use this form only for commercial members when NaviNet cannot be used, including:
- Out‑of‑network or out‑of‑state services
- Hospice
- Newborns not yet added to the plan
- Out‑of‑state FEP members
- When member information cannot be located in NaviNet
Thank you for your continued partnership in ensuring timely and accurate preauthorization processing. We appreciate your support as we enhance our digital tools to better serve you and our members.
Important Information and Updates
Beginning Feb. 26, 2026, pharmacy preauthorization decision letters will be available directly in CoverMyMeds (CMM) for requests decisioned on or after that date. This enhancement gives providers faster access to decision information within the platform they already use.
What to know
- Letters for requests decisioned before Feb. 26 will not appear in CMM
- BCBSNE will continue faxing all decision letters to the ordering physician’s fax number on record
- The BCBSNE preauthorization reference number can be found on the faxed decision letter or through our automated IVR phone system. Please use these resources before contacting Customer Service
- We continue to follow the standard 72‑hour turnaround time for peer‑to‑peer requests
As part of our ongoing effort to simplify processes and reduce administrative burden for providers, BCBSNE is excited to announce a significant update: Beginning March 3, 2026, prior authorization requirements for non‑contrast CT scans will be eliminated across both Medicare Advantage and Commercial lines of business.
This change supports a more seamless experience for members and providers and reflects our continued commitment to thoughtfully reimagining preauthorization.
Why This Matters
- Faster access to care: Providers can schedule non‑contrast CT scans without waiting for approval
- Reduced administrative work: Less time spent on submitting and managing authorizations
- Supports our long‑term vision: We continue to focus on improving processes and removing barriers where appropriate
Important Clarification
This change applies only to non‑contrast CT scans.
If a prior authorization request is submitted for:
- CT with AND without contrast, or
- CT with contrast,
➡️ These services will still require prior authorization and medical review.
Rollout Timeline
- Feb. 23: All non‑contrast CT PAs will be approved
- Feb. 26: Code removal begins
- March 2: System updates completed
- March 3: Go‑live - No prior authorization required for non‑contrast CT scans
BCBSNE medical policy for treating obstructive sleep apnea (OSA) includes oral appliances as one of several covered options. Sleep apnea treatment must be preauthorized for BCBSNE members or the claim will be denied as provider liability.
Because dentists are best qualified to evaluate a patient’s suitability for an oral appliance and complete dental impressions, BCBSNE provides specific billing and coding guidelines for these services.
Key points for providers
- Sleep apnea treatment requires preauthorization or the claim will be provider liability.
- Dentists must use CDT Dental Procedure Codes from the NEtwork BLUE dental fee schedule when submitting a medical claim.
- Oral sleep devices must be billed under E0486.
- E0486 includes:
- Fitting
- Adjustments
- Any related services such as x-rays, impressions and morning aligners
- Related services should not be billed separately, as they are included in E0486.
The medical policy for sleep apnea treatment is VIII.8 “Medical management for obstructive sleep apnea,” available at MedicalPolicy.NebraskaBlue.com.
What’s Changing
- Patient account numbers will no longer be included on reconsideration or appeal letters
- Member ID prefixes will also be removed
- Letters will continue to include the claim number and member ID, which remain the required identifiers for processing
Why This Change Is Occurring
- These updates help streamline our systems
- This reduce the need for custom development for elements not aligned with standard claims processing
Beginning April 2026, we will align with our Medicare Advantage (MA) procedures regarding the number of reconsiderations allowed per claim.
Key Update
- A maximum of two (2) reconsideration requests will be accepted for any single claim.
- The determination issued after the second reconsideration is final and binding.
- No additional reconsideration requests beyond the second will be accepted, reviewed, or processed.
- Additionally, participating (PAR) providers must submit reconsiderations via NaviNet, paper submissions will not be accepted, reviewed, or processed.
- Reconsiderations of beyond two per single claim or those submitted via paper will not be accepted, reviewed, or processed on or after April 6, 2026.
This update ensures consistency in processing and supports timely resolution of provider claims. For full details, please refer to Policy GP‑X‑073.
A reminder that the Coordination of Benefits (COB) policy GP‑X‑009 was updated on December 19, 2025. This update clarifies that all providers, including Dental providers, must follow standard COB rules to determine which payer is primary.
What Providers Need to Know
- When BCBSNE is the primary payer:
Benefits are determined as if no other plan provided coverage. - When BCBSNE is the secondary payer:
Benefits are determined after the primary plan has issued its benefit determination. - Secondary payer impact:
When acting as the secondary payer, BCBSNE may reduce benefits based on the primary plan’s benefit responsibility. - More than one Blues Plan:
File the claim with the primary Blue plan first; once processed, submit the secondary claim with a copy of the primary EOB.
Please ensure your teams are reviewing the updated policy and applying the COB rules accordingly.
Blue Cross and Blue Shield of Nebraska (BCBSNE) is excited to announce an upcoming partnership with ProgenyHealth, a leader in Neonatal Care Management Services. Beginning May 4, 2026, ProgenyHealth will support BCBSNE in enhancing care for premature and medically complex newborns.
This collaboration will bring additional clinical expertise and streamlined care management to support providers, families and our smallest members.
As part of this transition, the process for notifying BCBSNE of NICU or special care nursery admissions will change starting May 4, 2026. Additional details and instructions will be shared closer to the launch date.
Thank you for your ongoing partnership in caring for BCBSNE members.
BCBSNE is committed to making processes easy and efficient for providers. The fastest and most reliable way to submit requests is through NaviNet, our secure online portal.
Please note:
Reconsiderations for PAR (participating) providers cannot be submitted by mail or fax. Requests sent by mail or fax will not be processed. To avoid delays, submit requests electronically via NaviNet.
For details and access to the form, visit our Provider Academy page, where you’ll find eLearnings and FAQs with guidance on correct submission options.
Thank you for your attention and collaboration as we implement this updated process.
Before presenting an Advanced Beneficiary Notice (ABN) to a member, providers must verify benefits with BCBSNE or the member’s plan. ABNs should not be used for services that are covered, as this can lead to unnecessary costs and member abrasion. Covered services vary by plan, so confirming benefits is essential before presenting an ABN.
When an ABN Is Required
An ABN or waiver must be signed before services are rendered if the service may be denied as not medically necessary, investigative, or not scientifically validated. Providers must also inform the member in writing of potential financial liability and estimated costs.
For Federal Employee Program (FEP) members, a signed ABN is mandatory. Documentation in medical records will not be accepted.
Balance Billing Is Prohibited
In-network providers cannot balance bill BCBSNE members for amounts beyond the BCBSNE allowance for covered services.
For complete details, review policy GP-X-006: Hold Harmless and Balance Billing.
BCBSNE has historically created Coordination of Benefits (COB) claims on behalf of providers when a member was covered by BCBS policies. With our new payment cycle changes, providers will now be responsible for submitting COB claims directly in accordance with the COB policy.
To avoid claim delays and post-payment adjustments, please follow the steps outlined in Coordination of Benefits - GP-X-009:
- If BCBSNE or another Blue plan is secondary: Include the other carrier’s name and address on the claim filed to BCBSNE.
- If BCBSNE or another Blue plan is secondary to a non-Blue plan: Submit a copy of the primary carrier’s Explanation of Benefits (EOB) with the claim.
- If more than one Blue plan is involved: File with the primary Blue plan first; then submit the secondary claim with a copy of the primary EOB.
These steps help ensure timely processing and reduce administrative costs. Thank you for your attention to this update and for your continued partnership.
Blue Cross and Blue Shield of Nebraska (BCBSNE) is updating our Peer-to-Peer procedure (GP-P-003) to reflect recent system enhancements and align with Nebraska Legislative Bill 77 (LB77).
We are reversing our previous decision to postpone the shortened peer-to-peer request window. Our systems can now support electronic delivery of denial reasons through our portal, enabling providers to take timely action.
Key Updates:
- Request Window: Providers will have 3 business days (72 hours) from the post denial determination to request a peer-to-peer review for denials deemed not medically necessary on prospective and concurrent reviews.
- Denial Access: Denial reasons will now be available in portal, eliminating delays caused by mailed notifications.
- Physician Availability: Providers must offer a 2-hour window for the peer-to-peer discussion.
- Missed Request Window: If a peer-to-peer is not requested within the 3-day window, the provider must the appeal process.
We appreciate your partnership as we continue to improve transparency and efficiency in the prior authorization process.
When patient care extends into the new year, following our Split-Year Claim Submission policy ensures your claims are not rejected, returned or delayed due to billing errors.
Starting in March 2026, new medical policies will go into effect for both Commercial and Medicare Advantage plans. In Feb. 2026, revisions to existing medical policies will also take effect. To ensure a smooth transition and minimize any disruption to your current processes, please review the updated policies below.
MA New Polices Effective 3/1/26
Amniotic Membrane and Amniotic Fluid
Policy number: M.14
Summary: Amniotic membrane is covered for specific eye and wound conditions (e.g., diabetic ulcers, corneal ulcers), but amniotic fluid injections are not medically necessary for any indication. Only specific products will be allowed; all others will be considered non-covered on CMS (01/01/2026).
Benign Prostate Hyperplasia
Policy Number: M.29
Summary: Covers specific minimally invasive treatments for BPH—such as prostatic urethral lift (Urolift), water vapor therapy (Rezum), and transurethral waterjet ablation—when strict clinical criteria are met, while deeming other procedures (e.g., PAE, cryoablation, HIFU) not medically necessary.
Single Chamber and Dual Chamber Permanent Cardiac Pacemaker and Defibrillators
Policy Number: M.30
Summary: Covers permanent pacemakers for symptomatic bradycardia and implantable cardioverter defibrillators (ICDs) for specific high-risk cardiac conditions per CMS NCD criteria, while excluding reversible causes and non-indicated uses.
Electrophysiology Testing and Cardiac Ablation
Policy Number: M.31
Summary: Coverage for electrophysiology (EP) testing and cardiac ablation is based on InterQual criteria to determine medical necessity for diagnostic and therapeutic procedures.
Non-Coronary Vascular Stents
Policy Number: M.32
Summary: Coverage for non-coronary vascular stent procedures is based on CMS LCD criteria (L35998 and A57590) to determine medical necessity for vascular interventions.
Cardiac Catheterization
Policy Number: M.33
Summary: Covers right, left, or combined heart catheterization when clinically indicated for diagnosis or treatment planning of cardiac conditions, while excluding non-indicated uses such as routine angioplasty or electrophysiologic studies.
Orthognathic Surgery
Policy Number: M.34
Summary: Covers orthognathic surgery for correction of skeletal deformities causing functional impairment when strict clinical criteria are met, and for obstructive sleep apnea after failure of CPAP; excludes cosmetic indications and procedures lacking proven effectiveness.
Urinary Incontinence Treatment
Policy Number: M.35
Summary: Allows periurethral bulking agent injections for stress urinary incontinence meeting specific criteria, while deeming adjustable balloon continence devices and other non-proven treatments not medically necessary.
Non-invasive Cerebrovascular and Peripheral Arterial Vascular Studies
Policy Number: M.36
Summary: Coverage for non-invasive cerebrovascular and peripheral arterial vascular studies follows CMS LCD criteria (L35753, A57592, L35761, A57593) to determine medical necessity for diagnostic imaging.
Echocardiogram, Transthoracic (TTE) and Transesophageal (TEE)
Policy Number: M.37
Summary: Echocardiogram coverage is based on InterQual criteria, with PA required for TEE but not for TTE, ensuring appropriate use for cardiac evaluation.
Percutaneous Coronary Intervention (PCI)
Policy Number: M.38
Summary: PCI procedures are covered when meeting CMS LCD L34761 criteria for medical necessity in coronary artery disease treatment.
Category III Codes
Policy Number: M.39
Summary: Category III procedures are generally considered experimental or not medically necessary unless proven safe, effective, and consistent with accepted medical standards.
Physical Medicine and Rehabilitation
Policy Number: M.40
Summary: Continued physical therapy beyond 12 visits requires prior authorization and documentation showing functional improvement, medical necessity, and inability to perform therapy independently.
Prostate Rectal Spacers
Policy Number: M.41
Summary: Covers prostate rectal spacers (e.g., SpaceOAR) for patients with localized prostate cancer undergoing hypofractionated radiation therapy when strict clinical criteria are met; not medically necessary if criteria are not met.
MA Revised Policies effective 3/1/26
Bioengineered Skin and Soft Tissue Substitutes
Policy Number: M.3
Summary: Covers bioengineered skin substitutes for diabetic foot ulcers, venous leg ulcers, and certain reconstructive or burn indications when strict LCD criteria and approved product lists are met; all other uses and products are considered non-covered. Only specific products will be allowed; all others will be considered non-covered on CMS (01/01/2026).
MA Cosmetic and Reconstructive Surgery (Codes Added)
Policy Number: M.5
Summary: Adds new codes for cosmetic and reconstructive procedures; coverage follows NCD 250.5, NCD 140.2, and LCD 39051 criteria for medical necessity.
MA Cosmetic and Reconstructive Surgery (Tissue Transfer Flaps)
Policy Number: M.5
Summary: Adds tissue transfer flap codes; coverage determined using InterQual criteria for reconstructive surgery indications.
MA Radiology
Policy Number: M.13
Summary: Radiology procedures require PA and follow InterQual, NCD, and LCD criteria for specific codes, including nuclear medicine and advanced imaging.
MA Procedures Following NCD, LCD or InterQual
Policy Number: M.15
Summary: Adds genetic testing, behavioral therapy, and device codes; coverage based on NCD and LCD guidelines for medical necessity.
Evolent Joint Surgeries
Policy Number: (No M-number provided)
Summary: Joint surgery codes require prior authorization, managed by Evolent, for hip, knee, and shoulder procedures.
Commercial New Policies effective 3/9/26
Non‑Urgent Air Ambulance Transport
Policy Number: I.217
Summary: Requires preauthorization for planned air ambulance transfers and is covered only when the patient needs acute inpatient care unavailable at the originating facility, the nearest capable receiving facility is used, and no safer lower‑intensity transport option (ground or commercial air) is appropriate; transports for convenience are not medically necessary.
Commercial Revised Policies effective 2/15/26
Computed Tomography Angiography (CTA)
Policy Number: IV.62
Summary: The use of noninvasive fractional flow reserve following a positive coronary computed tomography angiography (75580) may be considered medically necessary to guide decisions about the use of invasive coronary angiography in individuals with stable chest pain at intermediate risk of coronary artery disease (i.e., suspected or presumed stable ischemic disease). All other uses are considered investigational.
Bone Mineral Density Measurement
Policy Number: IV.78
Summary: Screening for osteoporosis using Quantitative Computed Tomography (QCT) (77078), Ultrasound Densitometry and/or Vertebral Fracture Densitometry (77085, 77086, 76777) are Investigational.
Amniotic Membrane and Amniotic Fluid
Policy Number: I.200
Summary: The use of amniotic membrane using the following products **(Affinity®, AmnioBand® Membrane, Biovance®, EpiCord®, EpiFix®, Grafix™, NuShield®) is considered scientifically validated for treating specific conditions. These include diabetic lower extremity ulcers, neurotrophic keratitis, corneal ulcers or melts, pterygium repair, Stevens-Johnson syndrome of the eye, persistent epithelial defects, and chronic venous ulcers that have not healed after more than four weeks of standard therapy.
Bioengineered Skin and Soft Tissue Substitutes
Policy Number: I.202
Summary: The use of bioengineered skin and soft tissue substitutes is considered scientifically validated for the following indications:
Breast reconstruction (including each of the following: AlloDerm®, Cortiva® [AlloMax™], DermACELL™, DermaMatrix™, FlexHD®, FlexHD® Pliable™) OR
Diabetic lower extremity ulcers (AlloPatch®a, Apligraf®, Dermagraft®, Integra® Omnigraft™ Dermal Regeneration Matrix (also known as Omnigraft™), and Integra Flowable Wound Matrix, mVASC®, TheraSkin®) OR
Venous insufficiency lower extremity ulcers (Apligraf®, Oasis™ Wound Matrix) OR
Dystrophic epidermolysis bullosa (OrCel™ (for the treatment of mitten-hand deformity when standard wound therapy has failed and when provided in accordance with the humanitarian device exemption [HDE] specifications of the U.S. Food and Drug Administration [FDA]) OR
Second- or third-degree burns (Epicel® (for the treatment of deep dermal or full-thickness burns comprising a total body surface area ≥30% when provided in accordance with the HDE specifications of the FDA), Integra® Dermal Regeneration Template.
Oscillatory Devices for Respiratory Disorders
Policy Number: VII.35
Summary: High-frequency chest wall oscillation and oscillatory PEP devices are covered for cystic fibrosis, primary ciliary dyskinesia, and bronchiectasis; all other uses, including Volara device, are investigational.
Surgeries for Obstructive Sleep Apnea
Policy Number: III.62
Summary: Covers unilateral hypoglossal nerve stimulation (e.g., Inspire®) for adults and adolescents with Down syndrome under strict clinical criteria; bilateral hypoglossal nerve stimulation (e.g., Genio Device) is considered investigational.
Notifications about medical policy updates can be found under the “Recent Updates” section on the Alerts and Updates page.
Blue Cross and Blue Shield of Nebraska is simplifying the way we share information with providers. To help you stay informed without overwhelming your inbox, we’re moving to a quarterly publication schedule while continuing to deliver the same high-quality updates you depend on.
What’s changing?
Effective January 2026, both of our provider publications will be issued quarterly:
- Provider Bulletin – now published four times per year
- Provider Update Newsletter – also moving to a quarterly format
This adjustment reduces the number of separate communications you receive while ensuring you still get timely, relevant information in a more streamlined way.
What stays the same?
You’ll continue to receive:
- Actionable updates on policies, procedures and operational changes
- Notifications that support your day-to-day interactions with our plans
- Timely alerts posted to our online bulletin boards: Happening Now and NaviNet® Plan Central
We remain committed to making provider communications efficient and impactful so you can stay informed with confidence.
To streamline administrative workflows, improve processing timeliness and enhance overall care delivery efficiency, BCBSNE will transition to accepting pharmacy drug preauthorization requests exclusively through our digital tools starting Dec. 15, 2025. This change applies to pharmacy preauthorizations for our commercial lines of business.
What’s Changing?
BCBSNE is making it easier for providers to manage drug prior authorizations (PAs) with improved experiences available through CoverMyMeds. Key benefits include:
- Real-time eligibility checks to confirm coverage before submitting
- Electronic PA submissions - no more faxing paper forms
- Immediate confirmation of receipt - no need to call or resubmit
- Faster processing and reduced administrative burden
How to Submit?
- Participating providers: Use CoverMyMeds via NaviNet, our provider portal.
- Out-of-network or providers outside Nebraska: If you do not have access to CoverMyMeds via NaviNet, you may access CoverMyMeds through the Providers Preauthorization page.
Action Items:
- Register for NaviNet if you haven’t already
- Continue monitoring Happening Now and our Provider Bulletin for updates
Jan. 5, 2026 – Pharmacy Drug Prior authorization status will only be available using digital tools
- CoverMyMeds (if the request was submitted through CoverMyMeds)
- Online preauthorization platform, if the form was used
- Automated phone system (CSC will not be available)
Fax Line Retirement
Effective Dec. 15, 2025, the following prior authorization fax numbers will be retired:
- Pharmacy: 1-877-232-6726 or 402-548-4683
Important:
- Faxes submitted before Dec. 15 will receive a response notifying you of the upcoming change.
- After Dec. 15, commercial pharmacy preauthorization requests sent to any BCBSNE fax number will not be processed. Providers will receive a faxed response indicating the request was misrouted.
What’s Not Changing
- Medical Pharmacy prior authorizations will not be affected by this change. You may refer to the Medical Pharmacy Prior Authorization FAQs for additional information.
- The submission process for the following forms remain unchanged (though updates are planned later):
- Bowel Prep Cost Share Reduction
- Contraceptive Out-of-Pocket Reduction
- Dispense as Written – Prescriber Indicated Penalty Waiver Form
- ACA Formulary Exception Form
- NetResults Formulary Exception Form
- HMG Co-A Reductase Inhibitor (Statin) Cost Share Reduction
- HIV infection: Pre-exposure Prophylaxis (prep) medications
- Risk Reduction for Primary Breast Cancer in Women
Watch Happening Now for updates to these forms and our Pharmacy Management page.
We appreciate your partnership as we move toward more efficient, digital-first solutions to support you and your patients.
Blue Cross and Blue Shield of Nebraska (BCBSNE) has officially launched CoverMyMeds to simplify and accelerate the drug prior authorization (PA) process for providers.
What’s now available?
- Real-time eligibility checks to confirm coverage before submitting
- Electronic PA submissions – eliminating the need for faxing paper forms
- Immediate confirmation of receipt – no need to call or resubmit
- Faster processing and reduced administrative burden
These enhancements reflect BCBSNE’s ongoing commitment to reimagining preauthorizations and improving the provider experience.
BCBSNE is committed to making prior authorization easy and efficient for providers. The fastest and most reliable way to submit requests is through NaviNet, our secure online portal.
Please note:
In accordance with Nebraska LB77, medical Commercial prior authorizations cannot be submitted by mail. Requests sent by mail will not be processed. To avoid delays, submit requests electronically via NaviNet.
In limited cases, you may use the Universal Prior Authorization Request Form. This option is only for specific exceptions, such as:
- Hospice services
- Newborns not yet added to the benefit plan
- Member lookup issues
- Out-of-network or out-of-state providers without NaviNet access
For details and access to the form, visit our Preauthorization page, where you’ll find clear guidance and links to the correct submission options.
Thank you for your attention and collaboration as we implement this updated process.
As part of our ongoing efforts to support providers with the enhanced authorization submission process, we’ve created a series of short eLearnings to guide you through the updated workflows.
These modules cover both preauthorization and precertification processes for commercial, ACA and Medicare Advantage (MA) members, including behavioral health, home health and post-acute services.
Available eLearnings:
- Preauthorization
- General Preauthorization
- Behavioral Health
- Home Health
- Precertification
- General Precertification
- Behavioral Health
- Post-Acute SNF and Swing Bed
As we work through the enhancements, it's important “Urgent” priority type is used correctly. Please remember that “Urgent” should only be selected when a delay in care could result in serious harm to the patient’s health, safety, or bodily function.
Using “Urgent” incorrectly can delay processing for truly critical cases and impact overall workflow efficiency. If the case does not meet the criteria above, please select the appropriate non-urgent or retrospective priority instead.
These resources are designed to help you navigate the portal efficiently and understand when immediate approvals may apply.
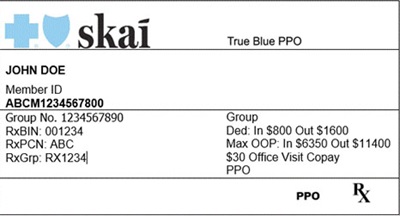
Effective January 1, 2026, Arkansas Blue Cross Blue Shield will introduce a new brand for their National Account members, including those affiliated with Walmart and other Arkansas-based national employers.
These members will now carry insurance cards branded as Skai Blue. A press release announcing this change was issued on October 24, 2025.
What Providers Need to Know:
- Skai Blue is an approved brand under Arkansas Blue Cross Blue Shield.
- These members may present cards that look different from traditional Blue Cross Blue Shield of Nebraska cards.
- Please treat Skai Blue cards as you would any other out-of-state Blue Cross Blue Shield card.
- Always validate eligibility and benefits by calling the number on the back of the member’s card.
An example of the new card is below:
At BCBSNE, we are committed to helping our members with complex medical conditions receive the care they need in the most cost-effective manner.
Starting Jan. 1, 2026, we will be removing Humira and Stelara from the following prescription drug lists: NetResults Performance, TraditionalRxList, ValueRxList and BluePride RxChoices. There are multiple biosimilar options available which are, in many cases, interchangeable with Humira or Stelara and will work the same.
Affected medications:
- Humira (adalimumab) and Stelara (ustekinumab)
Preferred biosimilar products:
- Humira:
- Adalimumab-aaty
- Adalimumab-adaz
- Hadlima
- Simlandi
- Stelara:
- Selarsdi
- Steqeyma
- Yesintek
Implementation details:
- Starting Jan. 1, 2026: Patients currently on Humira or Stelara will need to switch to a preferred biosimilar alternative for treatment.
- New therapy patients: Patients new to therapy will need to use a preferred biosimilar agent, per policy.
- Preauthorizations: Current preauthorizations extending beyond Jan. 1, 2026, will be transitioned to the biosimilar equivalent medication. Upon expiration, a new preauthorization request for the biosimilar medication will be required as is required today.
For questions regarding coverage, please refer BCBSNE members to call Member Services at the number on the back of their ID card.
Note: These changes do not apply to MA members or members using other BCBSNE prescription drug lists.)